Killer mold reveals its romantic side
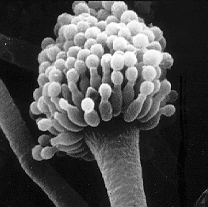
Take a deep breath. Now, don’t panic, but unless you live in a sterile chamber, you just breathed in between 1 and 100 spores of a killer mold called Aspergillus fumigatus. The spores are lightweight, microscopic, and everywhere – there’s literally no escaping them. However, if your immune system is intact (and I sincerely, absolutely hope that is the case), you need not fear for your life. The spores you just inhaled will be efficiently attacked by your innate immune system, which will render them dead as a doornail. And rest assured that this microscopic drama will continue to occur every day of your life, as it has for as long as you’ve lived.
Unfortunately, this is not always the case for HIV patients in advanced stages of AIDS, and cancer patients or organ transplant patients who are on high doses of immunosuppressants. For these groups, Aspergillus fumigatus can be deadly. It is one of the most frequent causes of hospital-acquired fungal infections. When it takes hold, it can invade the lungs or even other areas of the body, infiltrating through delicate tissue and causing terrific damage. Only a handful of anti-fungal medications are effective. We desperately need more in our arsenal, because some of the existing ones can be dangerous to the kidneys. With a mortality rate of over 50%, invasive aspergillosis is of grave concern.
I spent two years studying Aspergillus fumigatus while I was a postdoc in Nancy Keller’s laboratory at UW. Fungal biology was a new field to me; all of my previous research was in plant biology. We tried to understand how the mold produced certain toxins. Although A. fumigatus is a pathogen, researchers blessed with healthy immune systems (meaning, fortunately, our whole lab) do not have to take extensive protective measures to work on it, although we always used equipment called a laminar flow hood and wielded plenty of ethanol and bleach.
But one of the most frustrating things was that, unlike some of its relatives (including the innocuous model species Aspergillus nidulans), Aspergillus fumigatus had no known sexual stage. That meant we couldn’t use any time-saving classical genetic approaches when we worked on “fumi”, as we sometimes called it. Every time you wanted to study the effect of a particular gene, you had to artificially delete it by a laboratory method: generating naked protoplasts by digesting away the cell wall of spores, then bathing the protoplasts in DNA that had bits of the gene you wanted to delete flanking a “marker” (a gene that would allow transformants to survive on selectable nutrient media). This would ensure that the protoplasts would take up the replacement DNA, insert it in place of the existing gene. The resulting transformed mold strains would survive on the selectable media while those that weren’t transformed would not, since they didn’t have the marker gene. The “gene knock-out” process itself took an entire day, plus whatever length of time it took you to generate the necessary DNA, and screening for the desired strains took days to weeks. Progress was much faster with the sexually reproducing Aspergillus species, because if you found a mutant you were interested in, you could combine that mutation with another one by a simple sexual cross.
Fungal species for which the sexual stage is unknown are called “Fungi Imperfecti”. This used to be a formal taxonomic designation but is now used more loosely to describe sex-starved fungi. Just because a sexual stage hasn’t been discovered, of course, doesn’t mean that it won’t be. Some imperfect fungi do reproduce sexually – but may do so rarely or under conditions that are very hard to replicate in the laboratory. When a sexual stage is discovered, however, it is a big deal. A new Latin name is assigned to the sexual form, and it is fully documented in academic journals. With luck, scientists begin to understand what critical environmental factors are needed for the fungus to become, well, amorous.
A new article released online ahead of print in Nature describes exactly this. Three researchers - Celine M. O’Gorman, Hubert T. Fuller, and Paul S. Dyer of the University of Nottingham in the UK – hit the Aspergillus sexual jackpot. After collecting 91 isolates of Aspergillus fumigatus from soils in Ireland, the scientists did what many had tried before: they placed spores of different pairs of isolates on either side of nutrient-filled petri plates, used several different types of nutrient media, placed the plates in an incubator in the dark and hoped for the best. (No word on whether they played Barry White.)
Six months later, they saw something that no other humans in the world had ever seen before. On the plates containing oatmeal agar media, sexual A. fumigatus fruiting bodies had formed. Glistening spherical cleistothecia emerged in the margin between two compatible strains, the product of their union. As with other studied Aspergillus species, inside the cleistothecia were tucked ascospores, or the progeny. The researchers were able successfully cajole the ascospores into germinating, resulting in fungal baby photos that will be shown for decades to come.
So what does this all mean? Well, as a practical measure, it should speed up the pace of research on Aspergillus fumigatus – which is very much needed considering the impact of invasive aspergillosis on human health. It also helps us understand the biology of this fungus more. This mold is a major component of soils and compost, and plays an important role in recycling nutrients in the environment. We need to know how it grows, adapts, evolves, and genetically recombines to understand its place in the world – even as we try to make sure that our own lungs are not its rightful home.
In this respect, though, confirming that Aspergillus fumigatus reproduces sexually is fairly ominous. Resistance to the few antifungal medications we have available will emerge more quickly for a sexually reproducing species than one that only proliferates the less superior “parasexual” cycle that some mold species have, or by asexual reproduction.
Some years ago, I attended a social function at the UW Department of Medicine and happened to be seated by an oncologist. When I told him I was doing research on aspergillus, his face turned doleful. With the grim pragmaticism of one who has lost far too many patients to a complication, he said simply, “I hate aspergillus.” I can only hope that this and other research articles to come aid his efforts to keep this killer mold from harming more patients.
The scanning electron image of an Aspergillus fumigatus conidiophore was made available by the National Institutes of Health. One of the best information sources on the web for patients, medical professionals, and researchers alike is The Aspergillus/Aspergillosis Website at http://www.aspergillus.org.uk/.
Though a more modest contribution to the Aspergillus fumigatus literature, the final article resulting from my postdoctoral research was recently published in the journal Eukaryotic Cell. It focused on a gene and protein involved in chromatin remodeling; a topic for another post….


0 Comments:
Post a Comment
<< Home